

Different kinds of metal salts produce different colors in fireworks. Image via NASA.
On Wednesday, July 1, 2020, Canadians celebrated Canada Day, and Saturday, July 4 is Independence Day – commonly referred to as the Fourth of July – in the United States. And that means – whether you love or hate it – it’s fireworks season in North America.
For fireworks (a type of pyrotechnics) enthusiasts, the red, orange, yellow, green, blue and purple colors exploding in the skies create a lot of “ohhhhs” and “ahhhhs.” But what actually creates those brilliant colors?
As might be expected, science has the answer and it is simple: pure chemistry. The beautiful colors in fireworks are created by the use of metal salts. These salts are different from table salt, and in chemistry ‘salt’ refers to any compound that contains metal and non-metal atoms. Some of these compounds produce intense colors when they are burned, which makes them ideal for fireworks. Others, like potassium nitrate, sulfur and charcoal are often used to help the fireworks burn. Nitrates, chlorates and perchlorates provide oxygen for the combustion of the fuel. Dextrin, often used as a starch, holds the mixture together. Some colors can be strengthened with the use of chlorine donors.
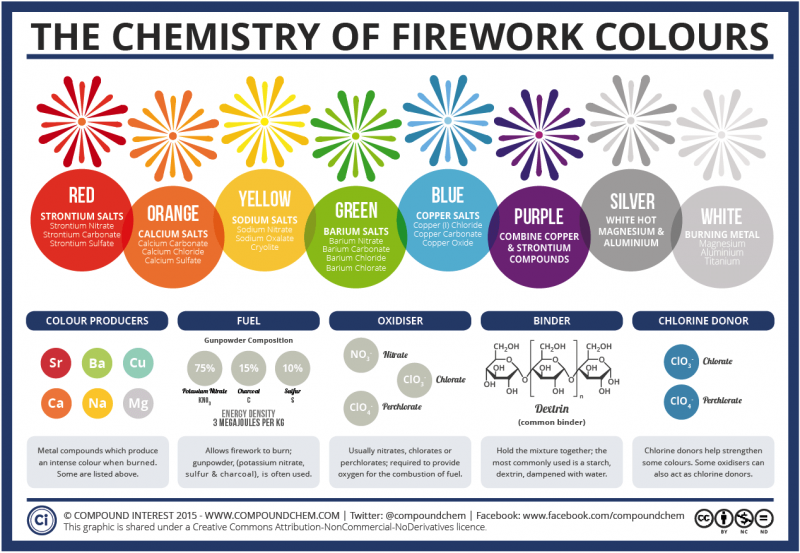
A more detailed overview as to how fireworks get their vibrant colors. Image via Compound Interest 2015.
Metal salts commonly used in firework displays include: strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks). Purple fireworks are typically produced by use of a mixture of strontium (red) and copper (blue) compounds.
The metal salts are packed into small pea- to plum-sized pellets called “stars” or “pyrotechnic stars.”
After a firework is ignited, a lift charge propels it into the sky. That’s just explosive black powder in a confined space that, when lit, causes a fast increase of heat and gas that can send a firework as high as 1,000 feet (300 meters) into the air.
Meanwhile, a time-delay fuse burns slowly into the interior of the firework shell. After about 5 seconds, as the shell is soaring overhead, the fuse kindles a charge that reaches the core of the firework, explodes and ignites the stars that contain the metal salts.

Fireworks in Austin, Texas, on July 4, 2013. Photo via our friend Sergio Garcia Rill.
Voila! A beautiful and colorful fireworks display.
By the way, the people who create fireworks are precise, expert craftsmen. Putting on a fireworks display is a complex process, and must be done safely. If even one thing is off — too much black powder, stars that aren’t aligned correctly or a trigger that fires too soon or too late — can cause everything else to go wrong.
Bottom line: The red, orange, yellow, green, blue and purple colors exploding in the night sky during a fireworks festival are created by the use of metal salts.
Read more: The Chemistry of Fireworks Colors
Read more: The Chemistry of Fireworks
Enjoying EarthSky? Sign up for our free daily newsletter today!
from EarthSky https://ift.tt/2ZAbYW3


Different kinds of metal salts produce different colors in fireworks. Image via NASA.
On Wednesday, July 1, 2020, Canadians celebrated Canada Day, and Saturday, July 4 is Independence Day – commonly referred to as the Fourth of July – in the United States. And that means – whether you love or hate it – it’s fireworks season in North America.
For fireworks (a type of pyrotechnics) enthusiasts, the red, orange, yellow, green, blue and purple colors exploding in the skies create a lot of “ohhhhs” and “ahhhhs.” But what actually creates those brilliant colors?
As might be expected, science has the answer and it is simple: pure chemistry. The beautiful colors in fireworks are created by the use of metal salts. These salts are different from table salt, and in chemistry ‘salt’ refers to any compound that contains metal and non-metal atoms. Some of these compounds produce intense colors when they are burned, which makes them ideal for fireworks. Others, like potassium nitrate, sulfur and charcoal are often used to help the fireworks burn. Nitrates, chlorates and perchlorates provide oxygen for the combustion of the fuel. Dextrin, often used as a starch, holds the mixture together. Some colors can be strengthened with the use of chlorine donors.

A more detailed overview as to how fireworks get their vibrant colors. Image via Compound Interest 2015.
Metal salts commonly used in firework displays include: strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks). Purple fireworks are typically produced by use of a mixture of strontium (red) and copper (blue) compounds.
The metal salts are packed into small pea- to plum-sized pellets called “stars” or “pyrotechnic stars.”
After a firework is ignited, a lift charge propels it into the sky. That’s just explosive black powder in a confined space that, when lit, causes a fast increase of heat and gas that can send a firework as high as 1,000 feet (300 meters) into the air.
Meanwhile, a time-delay fuse burns slowly into the interior of the firework shell. After about 5 seconds, as the shell is soaring overhead, the fuse kindles a charge that reaches the core of the firework, explodes and ignites the stars that contain the metal salts.

Fireworks in Austin, Texas, on July 4, 2013. Photo via our friend Sergio Garcia Rill.
Voila! A beautiful and colorful fireworks display.
By the way, the people who create fireworks are precise, expert craftsmen. Putting on a fireworks display is a complex process, and must be done safely. If even one thing is off — too much black powder, stars that aren’t aligned correctly or a trigger that fires too soon or too late — can cause everything else to go wrong.
Bottom line: The red, orange, yellow, green, blue and purple colors exploding in the night sky during a fireworks festival are created by the use of metal salts.
Read more: The Chemistry of Fireworks Colors
Read more: The Chemistry of Fireworks
Enjoying EarthSky? Sign up for our free daily newsletter today!
from EarthSky https://ift.tt/2ZAbYW3

Aucun commentaire:
Enregistrer un commentaire